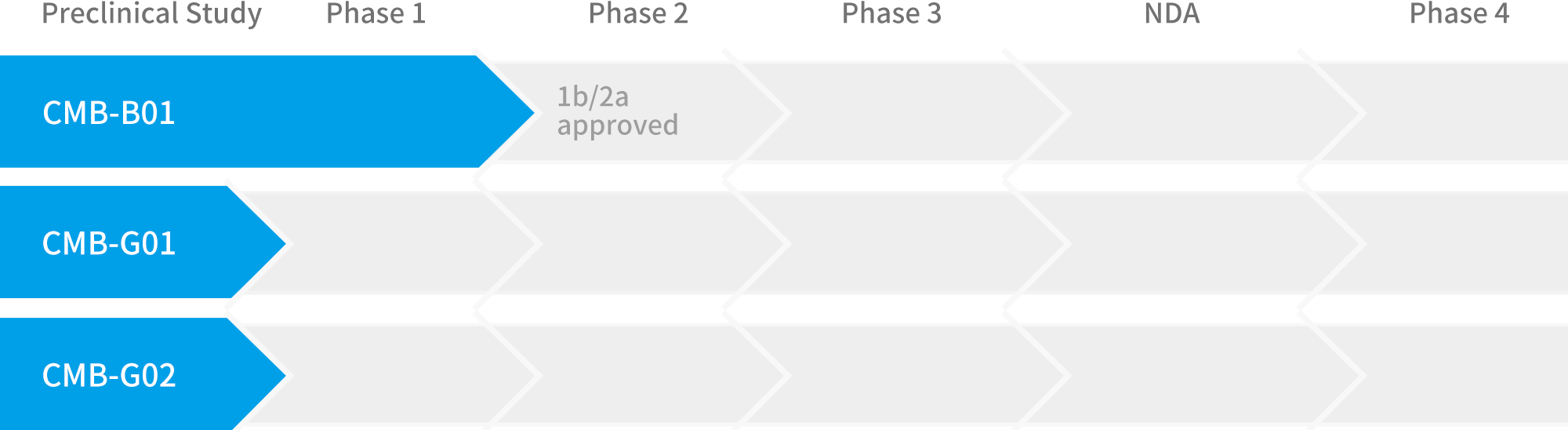

This project derives from the cancer research found by Academia Sinica. Through the efforts of Development Center for Biotechnology in manufacture and quality control technology development, the raw material medicine in this research has become a new clinical drug used to treat advanced melanoma. The clinical trial of this investigational new drug had the approval of US FDA in March 2017 (IND 133599). After the verification of series clinical trials in safety & efficacy, we vow to provide patients with a better choice of treatment.
Advanced melanoma is the deadliest form of skin cancer currently, it is also known as malignant melanoma, which is one of the relatively unpromising cancers.
1. Inhibits B16 melanoma growth in C57BL/6J mice.
2. Significantly inhibits NO production in vitro and in vivo.
3. Inhibits the enzymatic activity and protein expression of COX-2 and PGE2 production.
4. Suppressing IKBa phosphorylation and degradation, prevents NFkB p65 nuclear translocation.
5. Synergistically inhibits tumor cells with 5-fluouracil or Epirubicin in vitro.
IND 133599 is cleared by US FDA, and currently in preparation to apply for Taiwan clinical trial.